Abstract
Pharmaceutical manufacturers face increasing pressure to manage deviations and Corrective and Preventive Actions (CAPAs) with greater speed, consistency, and regulatory rigor. Yet traditional deviation management remains labor-intensive and fragmented, limiting the ability to identify systemic issues or drive continuous improvement. This study explores the potential of advanced artificial intelligence (AI) to transform deviation management from a compliance-driven obligation into a proactive, knowledge-driven process that drives efficiency, product quality, and cost savings.
AI offers pharmaceutical organizations the capability to convert deviation management from a document-heavy and reactive process into a strategic enabler of quality and efficiency. AI systems function as a force multiplier for quality teams by scoring and improving report quality, mining historical data for statistical root causes, applying retrieval-augmented generation (RAG) to recommend precedent-based CAPAs, and detecting anomalies that signal emerging risks. These approaches collectively enhance the accuracy, traceability, and speed of investigations while embedding data-driven reasoning into quality and production operations.
This paper synthesizes recent advances from both research and industry to describe four complementary pathways to create a combinatorial AI framework for a more robust deviation management in pharma. The framework includes narrative quality scoring, statistical pattern analysis, RAG-based CAPA identification, and categorization with anomaly detection. Together, they form a unified framework for intelligent deviation management. The study findings demonstrate that AI can transform each deviation into a structured learning opportunity, accelerating root cause determination, strengthening CAPA effectiveness, and promoting continuous improvement. By enabling traceable, evidence-based, and predictive quality oversight, AI allows pharmaceutical manufacturers to evolve from manual, document-centric workflows toward a culture of proactive compliance and operational excellence.
Rethinking Deviation Management and Motivation for AI
Pharmaceutical manufacturing operates under strict regulatory requirements, demanding rigorous deviation management and thorough root cause analysis for any process anomalies. Each deviation (departure from approved procedures or expected results) must be investigated and documented, through detailed quality reports, often followed by determining the appropriate Corrective and Preventive Action. CAPA is a systematic quality process to identify, investigate, and resolve product or quality problems to prevent their recurrence. In practice, however, the quality of these reports can be inconsistent, leading to inefficient investigations. Research indicates that a large proportion of deviations are attributed to human error without sufficient analysis of underlying systemic causes. Academic reviews suggest that human factors contribute to approximately 85–90% of process deviations and nearly one-quarter of all quality faults in pharmaceutical operations, with many investigations concluding only a probable rather than a confirmed root cause (Moorkoth et al., 2025). Such superficial attributions can obscure procedural, training, or design weaknesses, allowing recurring issues to persist and undermine both compliance and continual improvement efforts.
The operational and regulatory consequences of poor deviation management are significant. Inefficient or inconclusive investigations can delay batch releases, increase manufacturing costs, and invite additional regulatory scrutiny. Public reports indicate that the cumulative cost of Good Manufacturing Practice (GMP) noncompliance, including remediation, system upgrades, and penalties, can reach hundreds of millions of dollars for large manufacturers (Ayd, 2017). Compounding these challenges, quality teams face mounting workloads, as most deviations are classified as minor while requiring the same procedural rigor as critical events. This results in administrative backlogs and investigative fatigue. Fragmented information systems and complex standard operating procedures (SOPs) further hinder the identification of cross-case trends and the sharing of institutional knowledge. In sum, current manual approaches to deviation management remain resource-intensive, inconsistent, and reactive, ultimately posing risks to operational efficiency and product quality, compliance, and sustainability.
AI and data analytics offer a promising opportunity to transform deviation management. By leveraging techniques in natural language processing (NLP), machine learning (ML), and knowledge retrieval, pharma companies aim to improve the consistency of deviation reports, expedite root cause analysis, and proactively prevent recurrences. The motivation is clear. As AI-assisted systems can strengthen deviation investigations through comprehensive documentation, improvements can be observed in the extraction of insights from historical data, identification of root causes, and CAPAs effectiveness.
State of the Art in AI for Deviation Management
Recent years have seen growing interest in applying artificial intelligence to pharmaceutical quality investigations and manufacturing operations. NLP has emerged as a pivotal technology in this domain, as deviation reports, batch records, and CAPA documentation are largely composed of descriptive, unstructured text. In recent years, advances in large language models (LLMs) have revolutionized the capabilities of NLP, enabling deeper contextual understanding, entity recognition, and automated knowledge extraction from complex narratives. In pharmaceutical settings, NLP models can support deviation trending, anomaly interpretation, and proactive quality oversight by uncovering latent relationships across manufacturing and quality data sources (Vora et al., 2023).
Recent academic studies have begun to systematically assess the performance of advanced language models in pharmaceutical quality contexts, providing empirical validation for many of their natural language understanding capabilities. These investigations show that LLMs can extract key entities and contextual details such as dates, sites, products, and root causes, from deviation reports. Although hallucinations underscore the need for human validation, these models demonstrate strong reasoning ability when interpreting investigation narratives. Complementary work using text embedding models highlight their strength in retrieving semantically similar deviations from large archives, illustrating how LLMs and embeddings together can automate text analysis, populate structured fields, and enhance situational awareness during investigations. These findings reinforce that LLMs can substantially improve information extraction and analytical consistency when appropriately validated and domain-grounded (Salami et al., 2025).
Building on these advances in text understanding, recent research has shifted toward integrating LLMs with structured quality data and reasoning frameworks. Studies propose AI-enabled systems that retrieve historical deviations, CAPA records, and SOPs to contextualize new investigations, often using knowledge graphs to link related information across reports and procedural documents (Bahr et al., 2025). This integration reflects the growing use of RAG methods in pharmaceutical quality management, where validated internal knowledge, such as prior deviations and regulatory guidance, is retrieved and synthesized into evidence-based recommendations. Academic evaluations demonstrate that such retrieval-informed architectures can enhance factual accuracy, analytical depth, and decision consistency across regulated biomedical and pharmaceutical domains (Álvaro & Barreda, 2025; Kim et al., 2024).
Advanced data integration and analytics platforms are broadening the scope of deviation management in pharmaceutical manufacturing. Modern AI-driven systems can consolidate data from diverse sources, such as batch records, laboratory information management systems (LIMS), and deviation logs, into unified analytical environments that streamline investigations. These tools increasingly support real-time monitoring with automated deviation detection, offer user-friendly interfaces for exploring correlations, and generate standardized investigation summaries to improve documentation consistency (Niazi, 2025). By merging structured process data with unstructured investigation narratives, these systems connect batch context, equipment parameters, and quality results to more effectively uncover root causes. Much of this capability stem from process control initiatives, which integrate real-time process monitoring and data analytics into manufacturing oversight. However, these quantitative frameworks complement language-model-based approaches by addressing the quantitative dimension of root cause analysis, such as detecting parameter drifts or environmental shifts that precede deviations. An effective AI framework for deviation management therefore requires integration across textual and numerical data domains, bridging the gap between shop floor information and quality documentation.
Regulators and industry observers increasingly emphasize that manufacturers must move beyond simplistic attributions of human error and adopt continuous, data-driven monitoring of systemic quality trends. Current regulatory discourse highlights the need for AI and machine learning systems in GMP environments to be validated within a risk-based life-cycle framework that ensures transparency, traceability, and ongoing performance oversight. The European Federation of Pharmaceutical Industries and Associations (EFPIA, 2024) has further underscored that AI can play a meaningful role in identifying root causes and supporting effective CAPA generation through pattern recognition across historical deviation data.
In parallel, Quality Management System (QMS) platforms are beginning to operationalize these regulatory expectations by embedding AI capabilities directly into their architectures. Modern QMS solutions apply AI to classify deviations, link related records, and generate draft CAPA forms for review, improving both accuracy and auditability. Case studies demonstrate that integrated AI-driven deviation workflows can reduce investigation time by 50–70% while producing standardized, data-backed CAPA recommendations (Klyushnichenko, 2025). In such implementations, NLP systems parse deviation reports to retrieve relevant historical cases; machine learning models analyze structured process data to rank probable root causes; and the system recommends corrective and preventive actions, auto-populating CAPA drafts for expert validation. This holistic concept, from deviation intake to CAPA finalization, illustrates how regulatory and technological developments are converging to define the emerging state of the art in deviation management.
In summary, the current state of the art reveals a clear convergence between academic research and industrial practice in applying AI to deviation and CAPA management. Core advancements include LLM- and NLP-based tools for extracting insights from narrative reports, statistical modeling for uncovering causal relationships in complex datasets, and intelligent retrieval systems for leveraging institutional knowledge for decision-making. These innovations are increasingly validated through pilot implementations and peer-reviewed studies within highly regulated pharmaceutical environment, underscoring the importance of consistency, transparency, and data integrity. Collectively, these advances mark a paradigm shift from manual documentation toward data-driven quality management, positioning AI as a core enabler of transparent and evidence-based root cause analysis.
Multi-Model AI-Driven Framework for Smarter Deviation Management
To address the challenges and goals identified, pharmaceutical manufacturers can adopt an integrated AI-driven framework that enhances both deviation management and root cause analysis. Rather than treating investigations as isolated exercises, this combinatorial AI framework enables a continuous, data-rich improvement cycle. Each AI component targets a distinct but complementary aspect of the deviation lifecycle, from report creation to CAPA assignment, ensuring consistency, traceability, and regulatory readiness. The key AI pathways include: (1) a deviation Report Quality Scoring system that evaluates completeness and linguistic clarity to improve documentation quality; (2) a Root Cause Identification module that clusters incidents, uncovers latent correlations, and predicts likely causal categories; (3) a retrieval-augmented analysis engine that identifies analogous historical deviations and subsequently recommends CAPAs by leveraging institutional knowledge through RAG Processing; and (4) a Deviation Categorization and Anomaly Detection component that classifies new cases and flags unusual or inconsistent reports for targeted review. Collectively, these pathways shift deviation management from a reactive, manual process to a proactive, learning-oriented system. They support faster investigations, more accurate root cause determination, and data-driven CAPA decisions while reinforcing compliance with GMP expectations. Figure 1 provides a high-level view of how these four pathways interconnect to form an AI-enabled deviation management ecosystem.
AI Pathways for Deviation Management
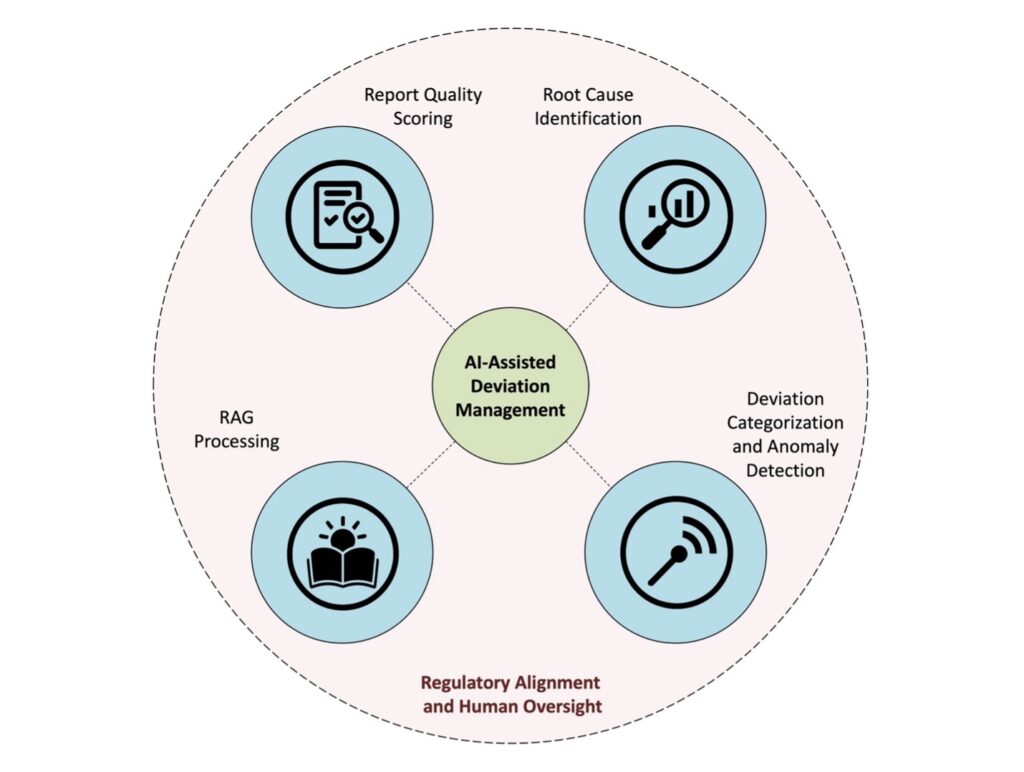
Figure 1. This figure illustrates four complementary mechanisms through which AI supports pharmaceutical deviation investigations. Report Quality Scoring ensures that deviation reports are complete, consistent, and audit ready. Root Cause Identification detects correlations and patterns in historical data, guiding investigators toward the most probable explanations. RAG Processing leverages institutional memory where past deviations and CAPAs are retrieved to provide precedent, and LLMs synthesize this knowledge into draft narratives and recommendations. Deviation Categorization and Anomaly Detection maintains classification consistency while surfacing unusual or exceptional cases for closer review. Together, these pathways form an integrated decision support system that strengthens compliance, accelerates investigations, and enhances organizational learning. By embedding Regulatory Alignment and Human Oversight around these pathways, the figure emphasizes that AI augments rather than replaces expert judgement, ensuring that deviation management remains both efficient and trustworthy.
Deviation Report Quality Scoring
High-quality deviation reports are foundational to effective deviation management. An AI-assisted Report Quality Scoring system can ensure that each investigation report is as complete and informative as possible. The idea is to use a combination of rule-based checks, LLM processing, and advanced NLP techniques to evaluate the completeness, consistency, and clarity of deviation writeups. The system would assign a quality score to each deviation report, accompanied by structured feedback to investigators, thereby promoting progressive improvements in documentation standards over time.
Key aspects of such a scoring system:
- Completeness of structured fields. The system should verify that all required fields in the deviation form, including date, batch, product, location, impact assessment, and immediate actions, are completed and that the entries are plausible. Missing or clearly incorrect information, such as placeholder text, would result in a lower quality score.
- Narrative content quality. Leveraging LLMs and advanced NLP techniques, the system can analyze the unstructured text descriptions of the event and investigation to ensure the presence of essential elements such as a clear statement of what occurred, the suspected root cause, investigation steps, and corrective actions. Beyond structural completeness, the model can assess whether human factors, such as training adequacy, fatigue, or procedural ambiguity, have been considered, whether the narrative appropriately links findings to the relevant SOPs and governing policies, whether product quality or patient safety risks are explicitly evaluated, and whether a plan for CAPA effectiveness verification is included. LLMs can also evaluate the narrative’s structure and information balance, ensuring that descriptions are complete, logically ordered, and aligned with investigation objectives. Reports with fragmented descriptions or missing contextual links can be flagged for refinement, helping to ensure that the investigation narrative is both coherent and comprehensive.
- Root cause depth and justification. The system should flag reports that rely on overly generic attributions without sufficient analysis or supporting evidence, since such explanations often obscure the underlying procedural, technical, or organizational causes. Using LLMs together with structured taxonomies, the system can evaluate whether an investigation demonstrates clear causal reasoning and adequate justification. It can assess whether the stated cause is supported by evidence (e.g., batch records, equipment logs, or laboratory results), whether corrective actions are balanced with preventive measures, and whether cross functional teams and recurrence awareness are reflected. By interpreting narrative logic and evidence consistency, the model assigns a root cause justification score, distinguishing reports that provide defensible, data-driven analysis from those that are superficial or incomplete.
- Consistency and language clarity. The system should ensure that investigation narratives maintain internal consistency and that CAPAs correspond precisely to the identified root causes. For example, an equipment-related issue should result in equipment focused corrective measures. Inconsistencies between the stated cause and corresponding actions indicate weaknesses in investigative quality. The model can further evaluate timeliness by checking whether investigations are initiated and closed within SOP defined timelines, as delays may reveal systemic inefficiencies. In addition, it can assess linguistic and regulatory compliance, flagging vague or non-audit-ready phrasing such as “probably” or “minor issue,” prompting investigators to adopt precise, evidence-based terminology. LLMs can also suggest clearer wording and highlight critical details that are insufficiently described. Collectively, these checks promote consistent, timely, and transparent communication of investigation findings in accordance with internal quality standards and external regulatory expectations. The Quality Scoring mechanism can generate a structured scorecard that provides both a quantitative quality score and targeted feedback, highlighting issues such as missing root cause statements or incomplete preventive actions. Calibrated against historical data, the system learns from reports associated with successful CAPAs and identifies patterns linked to recurring or reopened deviations. Integrated directly into the workflow, this real-time feedback helps investigators refine reports before submission, progressively improving documentation quality across the organization. Over time, the framework evolves into a continuous learning system that reinforces preventive practices and supports sustainable GMP compliance.
Root Cause Identification via Data Patterns
While higher quality deviation reports establish a stronger analytical foundation, AI can further enhance this capability by systematically examining historical data to infer probable root causes for new deviations. The Root Cause Identification module applies machine learning and data mining techniques to uncover latent patterns and cross variable relationships that may remain undetected through manual investigation. By leveraging large scale repositories of historical deviations and associated process data, such as equipment logs, environmental parameters, and laboratory results, the system can quantify associations between specific factors and deviation types, generating probabilistic estimates for potential root cause classes. This data-driven inference provides investigators with evidence supported hypotheses, accelerating root cause determination while improving reproducibility and analytical rigor across investigations.
Using text embeddings and categorical process features, the system groups historical deviation cases into clusters of semantically or contextually similar incidents. Within each cluster, the frequency distribution of known root causes can be used to estimate the likelihood of various explanations for a new deviation. For example, a model might determine that Cause A has a 60% probability, Cause B 30%, and Cause C 10%, enabling investigators to prioritize the most likely hypotheses while maintaining awareness of alternative possibilities. This transforms the clustering engine into a probabilistic decision-support tool rather than repeating deterministic classifications.
In more complex investigative contexts, advanced probabilistic modeling techniques such as Bayesian networks can be employed to capture causal interdependencies among diverse process variables. As demonstrated in recent biopharmaceutical studies, by integrating heterogeneous datasets, including deviation reports, CAPA records, environmental monitoring results, and equipment maintenance logs, AI systems can construct probabilistic graphs that map the relationships and conditional risk factors underlying deviations (Klyushnichenko, 2025). For example, a Bayesian network may reveal that a combination of a moderate rise in room temperature and a specific operator shift correlates with an increased probability of microbial contamination.
These models enable both retrospective and predictive insights. They not only clarify historical root causes but also identify emerging risk patterns before deviations fully manifest. For instance, if gradual increases in particulate counts within a filling suite have historically preceded deviations, the system can issue early alerts prompting proactive investigation. This transition from reactive analysis to predictive monitoring positions AI as a strategic tool for preventive quality assurance, allowing organizations to anticipate and mitigate potential deviations before they impact production or compliance.
It is important to emphasize that this ML module operates as an assistive decision support tool, providing data-driven insights rather than definitive conclusions. Investigators use the model’s probabilistic outputs and pattern detections to guide their analysis, but final root cause verification still depends on onsite evidence collection, such as equipment inspection, document review, and staff interviews. By narrowing the investigative scope and revealing relationships that may not be immediately apparent, this AI-assisted approach can substantially reduce the time and effort required for analysis. The system functions as a data-driven analytical assistant, rapidly synthesizing historical data to prioritize the most plausible explanations. As validated outcomes are reintegrated into the model’s training corpus, the statistical engine continuously refines its understanding of process dynamics and failure modes through ongoing feedback learning. This establishes a self-improving analytical ecosystem that strengthens both diagnostic accuracy and preventive foresight.
RAG-based CAPA Recommendations
A promising development in this area is the application of a RAG Processing module, which enable investigators to leverage prior cases and CAPA outcomes for faster and more consistent decision-making. In this approach, each new deviation is represented through advanced language model embeddings and compared against historical records to retrieve semantically similar and logically related cases. Retrieval serves as a structured mechanism for institutional knowledge transfer, grounding new investigations in verified historical evidence and minimizing duplication of analytical effort. Building on this foundation, generative AI synthesizes the retrieved knowledge into structured outputs such as draft investigation narratives or CAPA plans. When guided by verified evidence, LLMs can produce summaries and recommendations that are comprehensive, coherent, and aligned with regulatory expectations. Evidence from applications in other industries supports the potential of this method when applied to pharmaceutical manufacturing. In other studies, RAG systems have improved non-conformance handling in ceramics manufacturing by retrieving defect-specific data (Álvaro & Barreda, 2025), while knowledge-graph-enhanced RAG applied to Failure Mode and Effects Analysis (FMEA) has demonstrated superior factual accuracy and semantic reasoning beyond keyword-based methods (Bahr et al., 2025). In practice, AI-generated drafts remain subject to expert validation, ensuring adherence to GMP principles while expediting the preparation of investigation documentation.
Transparency and human oversight are integral to such systems. RAG frameworks can cite the specific deviation and CAPA records that inform each output, allowing investigators to verify the underlying evidence before approval. Integrated conversational interfaces further extend this capability, enabling users to query deviation data, process records, or highlight prior CAPA outcomes in natural language for rapid contextual insight. The RAG Processing module transforms historical deviation and CAPA data into an active decision support resource that preserves organizational knowledge, prevents recurrence of previously resolved issues, and enables faster, more consistent, and auditable decision-making in pharmaceutical deviation management.
Deviation Categorization and Anomaly Detection
Another pathway for strengthening deviation management lies in AI-based Deviation Categorization and Anomaly Detection. In regulated quality systems, deviations are typically classified by severity, type, or process area to support trend analysis and ensure consistent handling. AI tools can automate or verify classifications using historical, labeled data to map deviation narratives onto standardized taxonomies. For example, AI may attribute equipment failures to specific instrumentation or assign quality deviations to out-of-specification results. Consistent categorization improves data integrity, facilitates trend monitoring, and ensures that investigations are directed to the appropriate subject matter experts. The system can also act as a secondary control, flagging potential misclassifications. For example, identifying cases which have been labeled as manufacturing error that more closely align with laboratory testing deviations.
Beyond categorization, anomaly detection provides a complementary oversight by identifying exceptions rather than recurring patterns. Integrated into a multi-model quality system, it highlights deviations that fall outside expected clusters, signaling novel failure modes or irregular reporting practices. Examples include sudden increases in deviations linked to a specific shift, unusually brief narratives, or rapid closures lacking CAPA documentation. Anomaly detection can also reveal process or report inconsistences, such as persistently low-quality reports from a particular investigator or disproportionate deviations from a specific production line, which may indicate training needs or systemic weaknesses.
Together, Deviation Categorization and Anomaly Detection establishes a continuous quality feedback mechanism. They promote consistent classification, enable early recognition of atypical events, and generate actionable insights for preventive and corrective improvement across manufacturing operations.
Concluding Perspective
The convergence of AI and pharmaceutical quality management has the potential to make deviation investigations faster, more consistent, and more insightful. By adopting AI-assisted frameworks, pharmaceutical manufacturers can evolve from reactive, labor-intensive processes toward proactive, data-driven approaches to quality assurance. The multi-model framework presented in this paper illustrates how complementary AI components reinforce one another. Report Quality Scoring enhances documentation accuracy, machine learning identifies recurring patterns and probable root causes, RAG Processing connects new cases to organizational knowledge, and Deviation Categorization and Anomaly Detection ensures consistency while highlighting exceptional cases. Collectively, these components create an integrated foundation for the next generation deviation management.
Crucially, these AI-driven systems are designed to augment rather than replace human expertise. By automating repetitive data-gathering and documentation tasks, they allow investigators and quality engineers to focus on critical analysis, verification, and decision-making. AI contributes structured insights and evidence-based recommendations, while human experts provide contextual reasoning and regulatory accountability. This human-AI collaboration exemplifies GMP expectations for oversight and traceability while enhancing operational efficiency. As these systems continuously learn from both successful and ineffective CAPAs, their recommendations will grow more precise, establishing a feedback-driven cycle of organizational learning and continuous improvement.
In pharmaceutical manufacturing, where patient safety, compliance, and product quality are non-negotiable, the benefits of AI-driven deviation management are substantial. Organizations adopting these systems can expect more reliable root cause identification, shorter investigation cycles, and more consistent CAPAs, strengthening both compliance and operational performance. Ultimately, AI-assisted deviation management fosters a culture of quality where decisions are evidence-driven, investigations are thorough yet efficient, and lessons learned are systematically retained. This convergence of data, technology, and human judgement paves the way for robust processes, sustained regulatory compliance, and enduring assurance that every batch of medicine meets the highest standards of quality and control.
ABOUT ENTEFY
Entefy is an enterprise AI software company. Entefy’s patented, multisensory AI technology delivers on the promise of the intelligent enterprise, at unprecedented speed and scale.
Entefy products and services help organizations transform their legacy systems and business processes—everything from knowledge management to workflows, supply chain logistics, cybersecurity, data privacy, customer engagement, quality assurance, forecasting, and more. Entefy’s customers vary in size from SMEs to large global public companies across multiple industries including financial services, healthcare, retail, and manufacturing.
To leap ahead and future proof your business with Entefy’s breakthrough AI technologies, visit www.entefy.com or contact us at contact@entefy.com.
